Abstract
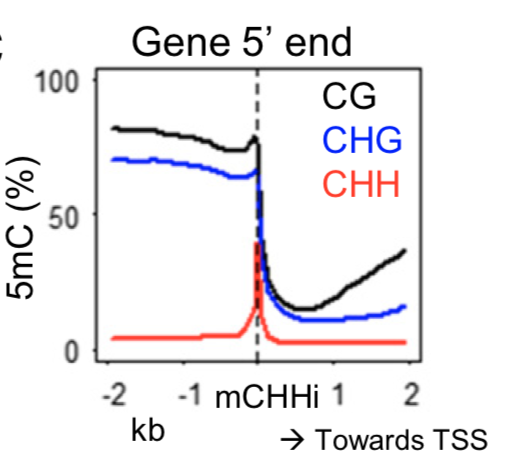
The maize genome is relatively large (∼ 2.3 Gb) and has a complex organization of interspersed genes and transposable elements, which necessitates frequent boundaries between different types of chromatin. The examination of maize genes and conserved noncoding sequences revealed that many of these are flanked by regions of elevated asymmetric CHH (where H is A, C, or T) methylation (termed mCHH islands). These mCHH islands are quite short (∼ 100 bp), are enriched near active genes, and often occur at the edge of the transposon that is located nearest to genes. The analysis of DNA methylation in other sequence contexts and several chromatin modifications revealed that mCHH islands mark the transition from heterochromatin-associated modifications to euchromatin-associated modifications. The presence of an mCHH island is fairly consistent in several distinct tissues that were surveyed but shows some variation among different haplotypes. The presence of insertion/deletions in promoters often influences the presence and position of an mCHH island. The mCHH islands are dependent upon RNA-directed DNA methylation activities and are lost in mop1 and mop3 mutants, but the nearby genes rarely exhibit altered expression levels. Instead, loss of an mCHH island is often accompanied by additional loss of DNA methylation in CG and CHG contexts associated with heterochromatin in nearby transposons. This suggests that mCHH islands and RNA-directed DNA methylation near maize genes may act to preserve the silencing of transposons from activity of nearby genes.